
Know Your Alcohols!
Published by Anne Altor on Mar 17th 2021
Know your Alcohols!
Not just cabernet from malbec, single malt from bourbon (although that's great too!) Alcohols are a diverse class of compounds. Some are liquid at room temperature, others are solid, some moisturize your skin and condition your hair, some are disinfectants! Here are some good reasons to learn about alcohols:
- To know if an ingredient is a moisturizer or will be drying
- To know if a product contains "hidden" conflict ingredient
- To demystify product labels
Read on for a primer on these diverse and (sometimes) delicious compounds!
What is an alcohol, anyway?
Chemically speaking, an alcohol is an organic compound that has an OH (hydroxyl) group attached to a saturated Carbon atom (a Carbon atom with no double bonds).
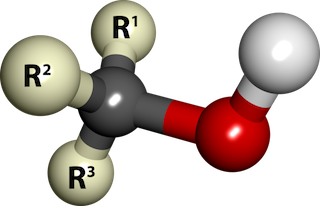
Ball-and-stick model of an alcohol molecule (R3COH). The red and white balls represent the hydroxyl group (-OH). The "R's" represent Carbon-Hydrogen groups. The grey ball is a Carbon atom. By SubDural12, Wikimedia Commons.
Many alcohols are made of small molecules, with chain lengths of 3 or fewer carbon atoms. For example, ethanol (CH3CH2OH) contains only 2 carbons. The OH group makes these small, liquid alcohols highly soluble in water.
Some alcohols have long carbon chains and are viscous or solid at room temperature. Take cetyl alcohol (an ingredient in our conditioner bars), for example (CH3(CH2)15OH). Long-chain alcohols are dominated by the hydrocarbon part of the chain. They look and feel more like fats or waxes than like what we may think of as a 'typical' alcohol. Compare the shape and size of ethanol and cetyl alcohol molecules below:
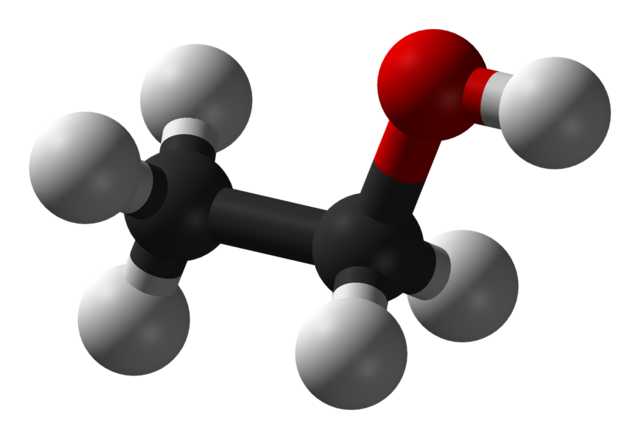
3D model of ethanol (CH3CH2OH) (Wikimedia Commons). The grey balls represent Hydrogen atoms, the black balls represent Carbon atoms, and the red ball indicates Oxygen. The Oxygen atom has a strong negative charge that makes this small molecule very soluble in water.
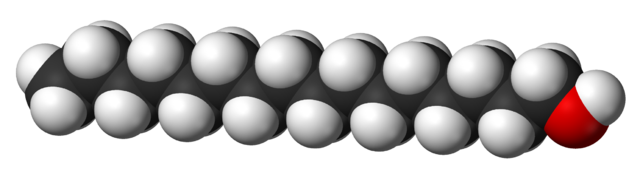
3D model of cetyl alcohol (CH3(CH2)15OH) (Wikimedia Commons). The color scheme is the same as for ethanol (the Carbons are on the inside of the model). Because the Carbon chain is so long, it dominates the behavior of this molecule and makes it much less soluble in water compared to alcohols with small molecular sizes.
Different Types of Alcohols
Alcohols are produced in plants, animals, microorganisms and fungi, and they play diverse metabolic and physiological roles. Some alcohols are produced industrially from organic molecules found in nature, including fossil fuels. Read on to learn about some common types of alcohols!
Fermented Alcohols

Fermentation vessels. Image by Tibor Janosi Mozes from Pixabay
Ethanol, probably the best known alcohol, is produced through fermentation. Fermentation is the microbial breakdown of sugars and starches in fruits, grains, sugarcane, and other organic materials. Ethanol (ethyl alcohol) can be distilled to remove water and other "impurities." Distillation concentrates the alcohol. Because it's small and has a charged part (the OH) and a non-charged part (the short Carbon chain), ethanol is a powerful and versatile solvent. It's soluble in water and can also dissolve nonpolar (noncharged) molecules.
Short-chain liquid alcohols like ethanol disrupt microbial cell walls, which makes them useful disinfectants. These alcohols are a primary ingredient in cleaning products including hand sanitizers. Ethanol is highly flammable and used as a fuel additive. This is controversial because ethanol decreases fuel efficiency and its production requires large amounts of energy and agricultural land.
Petroleum-Based Alcohols

Photo by Karolina Grabowska from Pexels
Some alcohols are derived from fossil fuels (petroleum), the most ancient organic matter on Earth. A prime example is isopropyl alcohol (IPA). IPA is produced from propane (natural gas). Isopropyl alcohol is a common disinfectant used in hand sanitizers and other cleaning products. IPA evaporates quickly without leaving a residue.
Fatty Alcohols

Photo by Sora Shimazaki from Pexels
Fatty alcohols are long-chain organic compounds produced by plants, animals and bacteria. Technically speaking they're aliphatic molecules (molecules without aromatic ring structures). Lipids and waxes in animals and plants contain fatty alcohols. Waxes and fats play many roles, including energy storage and protection of cells.
Fatty alcohols are widely used in skin and hair care cosmetics. A common example is cetyl alcohol, which we use in our conditioner bars. This compound was originally derived from whale oil, hence the name "cetyl" from Cetaceae, the Order encompassing aquatic mammals. Cetyl alcohol is now derived from coconut oil and palm oil. Ours is sourced from coconut.
Fatty alcohols are important conditioners and thickeners in skin and hair care products. They're emollients that soothe and soften skin, and occlusives that reduce water loss. These long-chain compounds are usually solid at room temperature and have a waxy texture. There is nothing drying about fatty alcohols.
Understanding alcohols and nomenclature can help you recognize conflict ingredients such as palm oil, which wears many disguises. Some fatty alcohols (including stearyl alcohol and some cetyl alcohol) are derived from palm oil. Although stearyl alcohol can be produced from coconut oil or tallow, much of it uses palm oil as its source. Cetearyl alcohol is a combination of cetyl and stearyl alcohols, so generally has palm oil as its source. Knowing this can help you avoid products with palm oil derivatives.
Sugar Alcohols

Image by 41330 from Pixabay
Alcohols with 3 or more OH groups are called polyols (alcohols with 2 OH groups are called diols). One class of polyols is the sugar alcohols, such as xylitol, sorbitol, glycerol, mannitol and erythryol. Sugar alcohols are produced by plants, algae and fungi. They're involved in plant metabolic processes and are a significant component of photosynthetic production.
Sugar alcohols are edible sweeteners. They contain fewer calories than glucose and sucrose, but they're not as digestible so can cause bloating and diarrhea.
The Diverse World of Alcohols
As you can see, alcohols are a diverse group of compounds that differ widely in structure and function. Understanding the basics empowers you to interpret ingredient labels and discriminate among product claims. Do you have questions we didn't answer about alcohols? Thoughts to share based on your experience? Leave a comment below to continue the conversation!
