How Natural Deodorant Works
Published by Anne Altor on Aug 24th 2023
To develop an effective formula for natural deodorant, it’s important to understand what causes body odor. This post shares some sweaty details about what causes BO and how natural deodorant works. Read on to learn about armpit ecology!
All about the pits
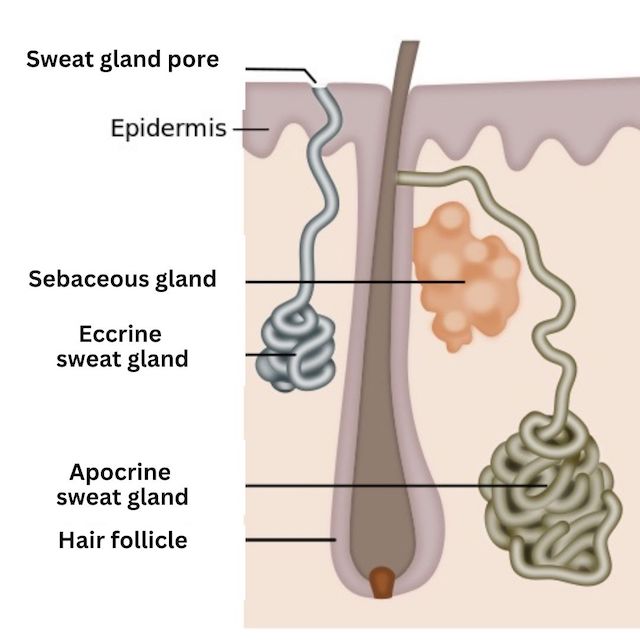
The armpit (“axillary”) environment is moist, warm and dark. Skin cells and sweat produced in the pits collect as organic matter, and this environment creates a great home for microorganisms (mainly bacteria and yeast). More on the microorganisms in a minute…
The armpits contain hair follicles and sweat glands, including apocrine and eccrine sweat glands. Each type of gland has a particular function.
Eccrine glands help to regulate body temperature. They secrete water containing salt ions (sodium, chloride, potassium, calcium & magnesium), amino acids, urea and other compounds. Apocrine glands are regulated by hormones and become active at puberty. Apocrine glands open into hair canals and secrete an oily mixture of proteins, lipids and steroids. These molecules are food for the bacteria that produce body odor.
As microorganisms consume and break down the organic molecules secreted by sweat glands, they release volatile odor compounds [2]. These odor-emitting compounds include volatile fatty acids (VFAs), such as hexanoic acids. Each person’s body odor is unique to them, as described by Ed Yong in I Contain Multitudes.

How antiperspirants work
Antiperspirants contain aluminum compounds that interact with proteins in sweat glands. When aluminum combines with protein, it creates a gel-like substance that plugs the glands and prevents sweat from escaping. That means any trapped sweat cannot release the waste materials it would otherwise carry out. Our sweat itself contains antimicrobial proteins that help regulate skin bacteria populations. Sweating releases wastes and helps regulate body temperature, both of which are important physiological functions.
Antiperspirants often contain perfumes, which cover over body odor. Some antiperspirants (and commercial deodorants) contain antibacterial compounds such as triclosan or propylene glycol. The widespread use of antibiotics has lead to the evolution of antibiotic-resistant bacteria and to imbalances in the natural human microbiome. Antiperspirants and deodorants that contain antibiotics cause changes in the armpit microbiome, and it’s not clear whether these changes are benign. One concern relates to possible links between aluminum in antiperspirants and breast cancer.
How natural deodorants work
Unlike antiperspirants, deodorants don’t plug the sweat glands. Natural deodorants use more gentle means to minimize odor, such as: 1) absorbing moisture to inhibit bacterial growth; 2) neutralizing odor-causing molecules (described below); 3) changing the pH to reduce bacterial growth; 3) using natural antimicrobial compounds like essential oils to reduce bacteria and enhance aroma.
Basic chemistry of odor neutralization:
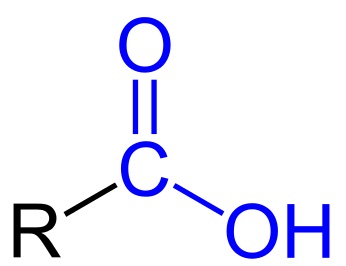
The odor-causing molecules produced in our armpits are a type of chemical compound called hexanoic acids. These weak carboxylic acids have a carbon compound (“R” in the equation below) attached to a carboxyl group (COOH).
These odor compounds don't dissolve readily in water, and they're volatile, which means they can vaporize and be picked up by our noses. When a compound like magnesium hydroxide is present, the hexanoic acid gets neutralized by forming a “salt”. The way this happens is that the acid releases a proton (H+), which leaves the acid with a negative charge. The positively charged magnesium from the magnesium hydroxide is attracted to the negatively charged hexanoic acid; the ionic compound that forms by this interaction is soluble in water, so it's not volatile and doesn't vaporize:
(R–COOH)2 + Mg(OH)2 → 2R–COO- Mg2+ + 2H2O
Ingredients in our natural deodorant
Here are the key ingredients in our deodorants, with a description of what they do:
Organic tapioca starch: Plant starches that absorb moisture and help keep the pits dry. They also help create a soft powdery feel.
Magnesium hydroxide (Mg(OH)2): A naturally occurring mineral that dissolves slowly in water. MgOH2 absorbs sweat, and as it slowly dissolves, it reacts with and neutralizes the odor-causing acids produced by bacteria. Magnesium hydroxide also slightly raises the underarm pH, which may make the environment less favorable to odor-causing bacteria.
Organic Fair-Trade coconut oil: Absorbs quickly, soothing, has natural anti-inflammatory properties.
Organic mango butter: From the mango pit to your pits! Organic mango butter soothes the skin and creates a thick, creamy texture to the deodorant. Along with coconut oil, it helps to protect the skin and spread the active ingredient (magnesium hydroxide) around the axillary area.
Organic fractionated coconut oil: The liquid portion of coconut oil after the saturated fatty acids have been removed. A very light, non greasy oil that helps to blend all of the ingredients together into a silky mixture that spreads evenly.
Sunflower wax: We add a small amount of sunflower wax to thicken the deodorant, adds emollience and help with spreadability.
Zinc ricinoleate: Zinc ricinoleate is derived from castor oil. It absorbs and thus "traps" odor-causing molecules, and it's also thought to have mild antimicrobial effects.
Essential oils: Liquid plant essences. Many essential oils have antimicrobial effects as well as aromatic properties. In deodorant, they help to reduce bacterial populations and create a lovely aroma that interacts with your own natural essence.
Baking soda vs. magnesium hydroxide in natural deodorant
Many natural deodorants use baking soda (sodium bicarbonate, NaHCO3) to neutralize odor-causing volatile fatty acids. However, baking soda dissolves quickly in water, quickly raises the armpit pH, and can be very irritating to the skin. In contrast, magnesium hydroxide is less soluble and so it dissolves slowly. This means that the change in pH is less dramatic, the duration of odor-neutralization is increased, and the formula is more gentle. We use only magnesium hydroxide, not baking soda, in our natural deodorant.